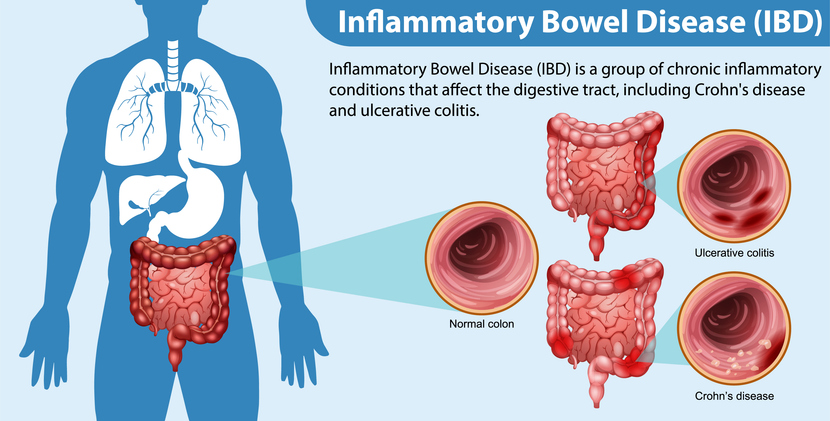
Ulcerative colitis (UC) is a type of inflammatory bowel disease (IBD) that causes inflammation and ulcers (sores) in the digestive tract. This condition predominantly affects the innermost layer of the large intestine, encompassing both the colon and rectum. In Ireland alone, at least 40,000 individuals live with IBD, contributing to a global tally of over 5 million. This widespread prevalence underscores the importance of research to understand better and manage this debilitating condition.
In a groundbreaking study published in Computers in Biology and Medicine, a collaborative effort between CÚRAM researchers at the University of Galway and their counterparts at the University of Birmingham has resulted in a pioneering computational model. This model simulates the distribution of shear stress in the colon, considering variations in mucus thickness. The colon, an essential digestive system component, employs rhythmic contractions to propel waste. These contractions generate mechanical forces that impact the mucus layer lining the colon, which serves as a critical barrier, isolating the body’s internal environment from countless gut microbes.
The novel computational model developed by the research team leverages actual data on colonic movements, mucus properties, and tissue characteristics to recreate this dynamic internal environment. The findings from this study suggest that the mucus layer functions as a lubricant, enhancing faecal velocity and facilitating the passage of waste through the colon. However, this lubricating effect is reduced in UC patients due to a thinner mucus layer, which may lead to constipation. This insight provides a clearer understanding of how UC symptoms develop and offers potential targets for therapeutic intervention.
The study underscores the mucus layer’s protective role, safeguarding the cellular layer responsible for colonic processes against mechanical stress. Led by Dr Yury Rochev from the School of Physics at the University of Galway, the team explored how shear stress differed across various functional zones within the colon. These variations could play a role in regulating cell migration, differentiation, and immune responses. A compromised protective mucus layer, as seen in UC, could lead to increased inflammation and tissue damage. Dr Rochev highlighted the significance of their findings, stating, “Our model shows that mucus significantly enhances faecal flow and eases the movement of waste in the colon, a mechanism that is less effective in ulcerative colitis due to mucus thinning, potentially leading to constipation.”
Moreover, the model sheds light on the mucus’s protective function, shielding the delicate cellular layer that manages essential colonic processes from mechanical forces generated during bowel movements. Dr Rochev further noted, “Our investigations into the mechanical stress distribution across the cellular layer reveal variations that could influence cellular behaviour, which, when disrupted, as in UC, may contribute to inflammation and tissue damage.” The research team is not solely relying on computational simulations; they are also developing an experimental model using “organ-on-a-chip” technology to validate their computational predictions.
Ibrahim Erbay, another researcher on the team, elaborated on their experimental approach: “We are employing intestinal organoids to replicate the thin cellular layer of the colonic surface. By circulating fluid within the organ-on-a-chip platform, we simulate the mechanical forces that are naturally present in the colon.” By integrating computational modelling with rigorous experimental validation, the researchers aim to achieve a detailed understanding of how mechanical forces affect biological processes in both health and disease. This comprehensive approach is expected to enhance our knowledge of gut health and lead to the development of novel, targeted treatments for inflammatory bowel diseases and other digestive disorders. Erbay added, “This research not only deepens our understanding of basic colonic functions at the cellular level but also provides a valuable tool for developing new therapeutic strategies. With our ability to model various drug delivery systems, we can optimise treatments, potentially improving outcomes for conditions affecting the gut.”
More information: I.H. Erbay et al, Computational insights into colonic motility: Mechanical role of mucus in homeostasis and inflammation, Computers in Biology and Medicine. DOI: 10.1016/j.compbiomed.2024.108540
Journal information: Computers in Biology and Medicine Provided by University of Galway