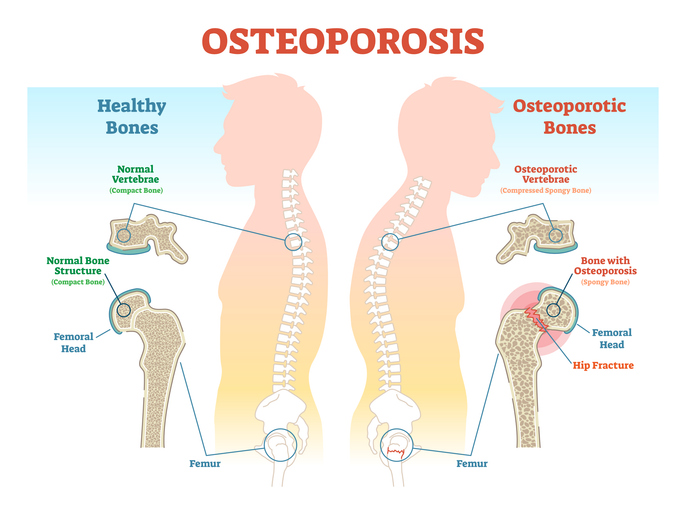
Osteoporosis, a condition characterized by weak and fragile bones, is a pressing global health issue. It leads to approximately 8.9 million fractures annually, with a fracture occurring every three seconds. The elderly are particularly susceptible, as ageing weakens bones, often necessitating long-term care. As the population ages, there is a growing urgency for healthcare systems to discover effective treatments for this condition.
One promising treatment involves using parathyroid hormone (PTH), specifically a drug called teriparatide, which helps strengthen bones by promoting bone formation. However, PTH can also stimulate the production of osteoclasts, cells that break down bone tissue, which can sometimes counteract its bone-building effects. Understanding how PTH works at a molecular level is crucial to improving osteoporosis treatments and ensuring better patient outcomes.
Recently, researchers at Tokyo University of Science, led by Professor Tadayoshi Hayata and Ms. Chisato Sampei, investigated genes PTH affects in bone-forming cells called osteoblasts. Their study, published in May 2024 in the Journal of Cellular Physiology, aimed to identify new targets for improving osteoporosis therapies. In Japan, where approximately 12.8 million people have osteoporosis, finding effective treatments is particularly urgent to enhance the quality of life.
Using experiments with mouse cells and live mice treated with teriparatide, the researchers used advanced techniques to study how PTH influences gene activity. They discovered a gene called Gprc5a that becomes more active when PTH signalling is triggered. Gprc5a codes for a protein involved in cell signalling and has just been well-studied in the context of bone health.
Interestingly, when the researchers suppressed Gprc5a in their experiments, they observed increased activity in genes related to bone cell growth and maturation. This suggests that Gprc5a normally slows down these processes, potentially limiting the effectiveness of bone-strengthening treatments like teriparatide. Understanding this could lead to new strategies for enhancing the effects of existing osteoporosis treatments.
Further investigation revealed that Gprc5a interacts with a protein receptor called Activin receptor-like kinase 3 (ALK3), part of a pathway that influences bone development. By affecting this pathway, Gprc5a appears to regulate how bones form and grow. These findings provide valuable insights into the complex processes that maintain bone strength and offer new avenues for developing more targeted osteoporosis therapies in the future.
Prof. Hayata and his team’s study illuminates how PTH influences bone health through genes like Gprc5a. By unravelling these molecular mechanisms, researchers aim to enhance treatments for osteoporosis, potentially leading to better outcomes for patients worldwide. This research represents a significant step towards personalized medicine approaches that could customize treatments to individual patient’s needs, ultimately improving their quality of life and reducing the burden of osteoporosis on healthcare systems.
More information: Chisato Sampei et al, Gprc5a is a novel parathyroid hormone-inducible gene and negatively regulates osteoblast proliferation and differentiation, Journal of Cellular Physiology. DOI: 10.1002/jcp.31297
Journal information: Journal of Cellular Physiology Provided by Tokyo University of Science